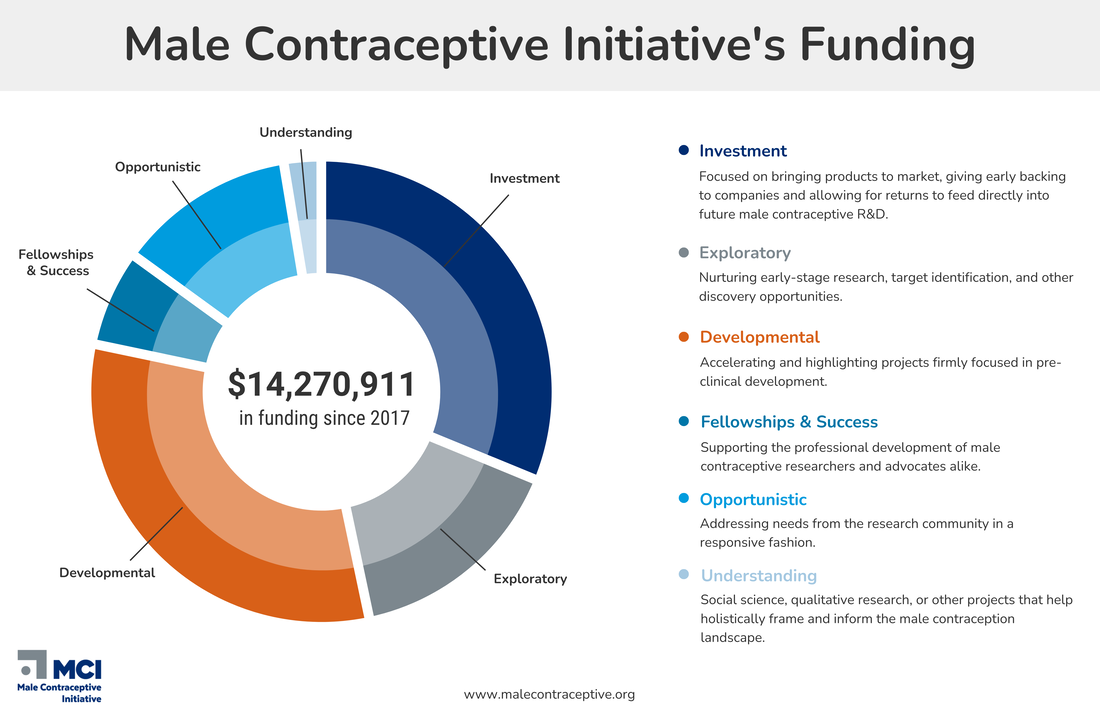
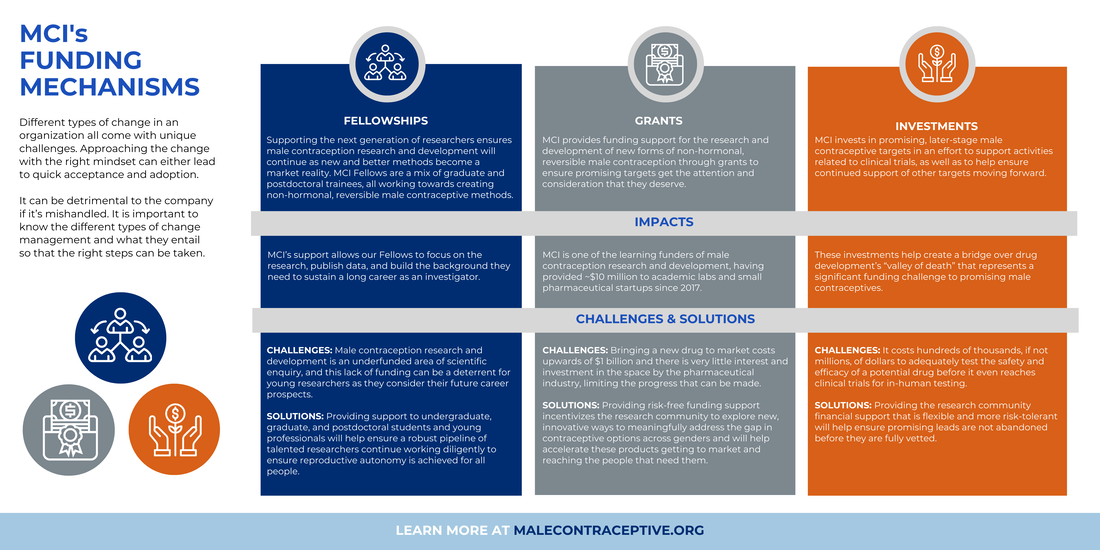
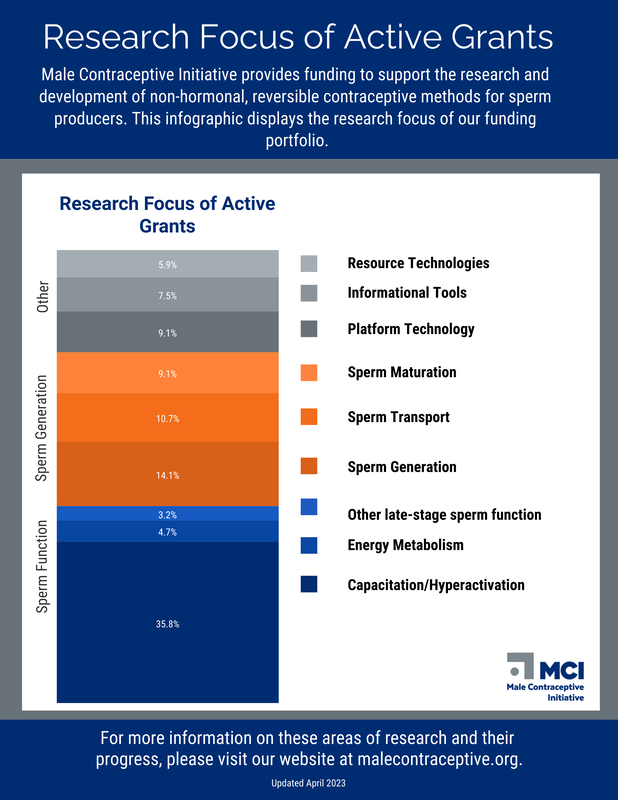
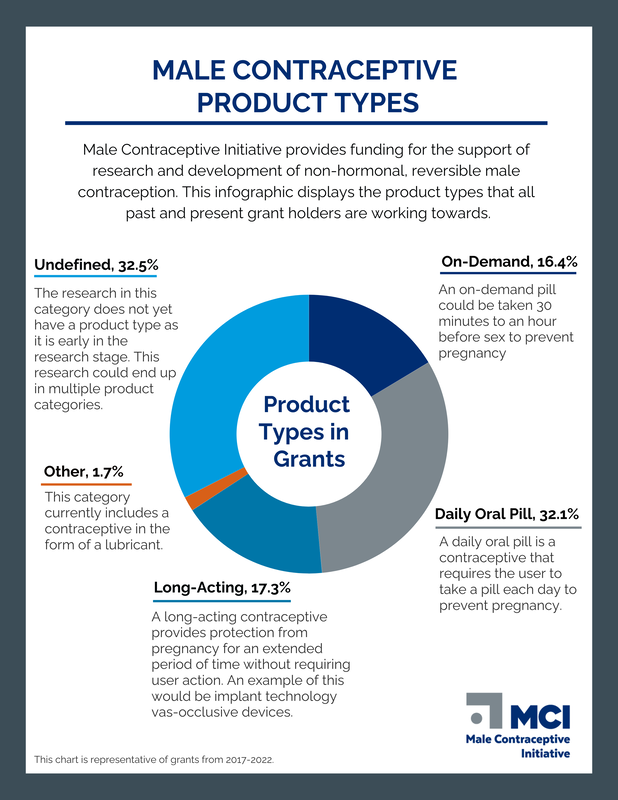
We provide funding support for the research and development of new non-hormonal, reversible male contraceptives.
Male Contraceptive Initiative supports science that moves male contraceptives forward. We support a broad program of research including discovery-stage and pre-clinical studies across the drug and device realms. We also support young researchers, development of tools that promote the field, and social science that illustrates the demand and need for male contraceptives. On this page we feature information about our funding schemes, links to internal and external announcements, and contact information.
|
Discovery and Development
These awards support promising development programs advancing towards first-in-man studies. |
Innovation Grants
These grants support exciting early stage research in previously unexplored areas – investing in the future. |
Other Funding
We look for innovative opportunities and investments in the field of male contraception research. |
Trainee Support
We support graduate and postdoctoral trainees, allowing them to grow their careers and build their own programs. |
|
At Male Contraceptive Initiative, we believe that new ideas can come to fruition in myriad ways. We support scientists, students, and researchers across the globe, working in academia, private industry, and nonprofits.
Our funding portfolio of long and short term investments in the field accelerate the development of contraceptives like devices that can last for years, on-demand contraceptives, or daily pills. Other areas of investment include market research, moonshot projects, and early career support for young investigators. Looking for funding opportunities? Click here. |
Male Contraceptive Initiative's Funding Strategy
|
What are the male birth control research activities is MCI supporting?
The science of male birth control can be a little intimidating if you do not have a background in science. To counter this, we created this section to make the work we are supporting a bit more accessible to the general public.
A single injection that ensures a man cannot cause a pregnancy for years and is completely reversible.
|
Our grantee Contraline has invented a proprietary hydrogel called ADAM™ that is implanted into the vas deferens through a minimally invasive, outpatient procedure.
The hydrogel works by blocking sperm from traveling through the vas deferens. Similar to intrauterine devices (IUDs) for women, Contraline’s contraceptive is designed to last for years and be reversible. MCI’s support will be used by Contraline to prepare for filing with the FDA and initiating first-in-human trials. |
A reversible male birth control that impacts the head of the sperm, preventing it from fertilizing an egg.
|
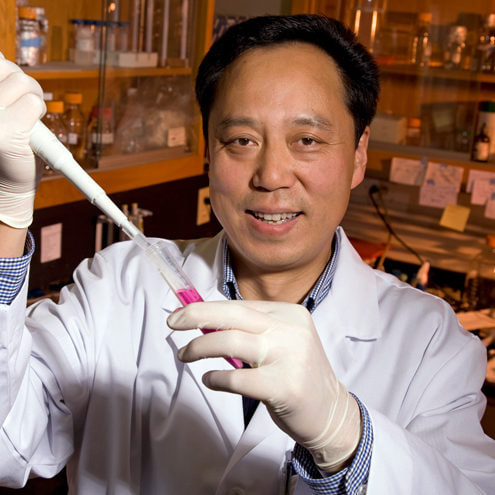
Our grantee Dr. Wei Yan and his team of researchers have developed a new compound derived from a Chinese herb called the Thunder God vine (Tripterygium Wilfordii Hook F).
The compound works by causing the head of sperm to be malformed such that they are not able to connect with and fertilize a female egg. This effect only occurs while the compound is consumed, and normal function is fully restored after discontinuing use. MCI’s support allowed Dr. Yan and his team to explore the effects of the compound and publish their results. Because of MCI’s grant, our sister organization Contraceptive Accelerator Network is performing nonclinical studies on Triptonide that advance it towards human studies. |
|
Our grantee Dr. Mario Buffone has been working in the field of sperm physiology for two decades. His lab seeks to understand the complex process of mammalian sperm capacitation, the change sperm undergo in the female reproductive tract that enables them to penetrate and fertilize an egg.
Dr. Buffone is working to screen and identify drugs that are able to prevent the capacitation and therefore render sperm incapable of fertilizing an egg. MCI is supporting the Buffone lab’s studies with the intent of moving towards in vivo preclinical studies. |
A biodegradable implant injected just below the skin’s surface that can deliver a male contraceptive over a sustained period of time.
|
Our grantee Dr. Rahima Benhabbour designs and fabricates delivery platforms for HIV prevention, cancer treatment, and contraception.
Dr. Benhabbour launched her first start-up AnelleO, Inc. in 2016, focusing on developing 3D-printed intravaginal rings for the treatment of infertility. MCI’s support will help facilitate studies that develop and implement an injectable device that can carry drug and translate into human studies. |
A daily, or even on-demand, method of male birth control that prevent sperm from being able to swim when taken.
|
Our grantee Dr. Gunda Georg’s group has published over 200 scientific articles on the design, synthesis, and evaluation of biologically active agents. In addition to male contraception, her lab investigates areas of cancer and female contraception.
Dr. Georg's lab has focused on investigating the testis-specific serine kinase (TSSK) family. These proteins are expressed after sperm have been developed, and are likely involved in multiple steps that could make them excellent contraceptive targets. MCI’s support will help Dr. Georg’s team investigate the sperm-specific isoform of Na,K-ATPase, which is crucial for sperm motility (e.g., sperm’s ability to swim). |
|
Our grantee Dr. Mike O’Rand is the President and Chief Scientific Officer of Eppin Pharma, named after the protein EPPIN which is a critical component of sperm development.
Dr. O’Rand is developing a compound called EP055, a small organic compound that that targets EPPIN on the surface of sperm and prevents sperm from swimming. MCI’s support will help support Dr. O’Rand Investigational New Drug (IND)-enabling milestones that are required for Eppin Pharma to begin the process of entering first-in-man studies for EP055. |
A reversible male birth control method that causes sperm to become sterile.
|
Our grantee Dr. Stephen L’Hernault and his lab have worked on spermatogenesis (i.e., sperm creation) and fertilization for over 30 years.
Dr. L’Hernault is working on developing compounds that can block the function of the IZUMO1 protein, which is required to fertilize an egg. MCI’s support will help Dr. L’Hernault’s team to identify small compounds that reversibly block the function of the IZUMO1 protein. |
A cohort of excited young researchers motivated by curiosity, ambition, and the social good associated with male birth control.
|
Our Fellowship program features a mix of graduate and postdoctoral trainees, all working towards creating non-hormonal, reversible male contraceptive methods.
Our Fellows work on a diverse portfolio of projects that help move the male birth control forward. Our support allows our distinguished Fellows to focus on the research, publish data, and build the background they need to sustain a long career as an investigator. |
An on-demand method of male birth control that you take just before sex.
|
Drs. Lonny Levin and Jochen Buck are professors at Weill Cornell Medicine and are working on inhibitors of soluble adenylyl cyclase (sAC) as on-demand male contraceptives. Their deep expertise in sAC keeps them attuned to the effects of inhibitors on sperm functions such as motility.
MCI is supporting work that develops novel inhibitors of sAC as on-demand male contraceptives as well as translational research that assesses the pharmacodynamics of inhibitors on human sperm. These steps are crucial to understanding how the drug stays associated to sperm after ejaculation and its potential contraceptive efficacy. |
Contraceptive Funding, Simplified