|
Introduction The drug development process is often presented as a linear process: you achieve one milestone before moving on to the next, with progress taking the developer ever forward. The Food and Drug Administration defines this process as such:
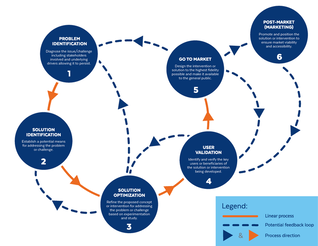
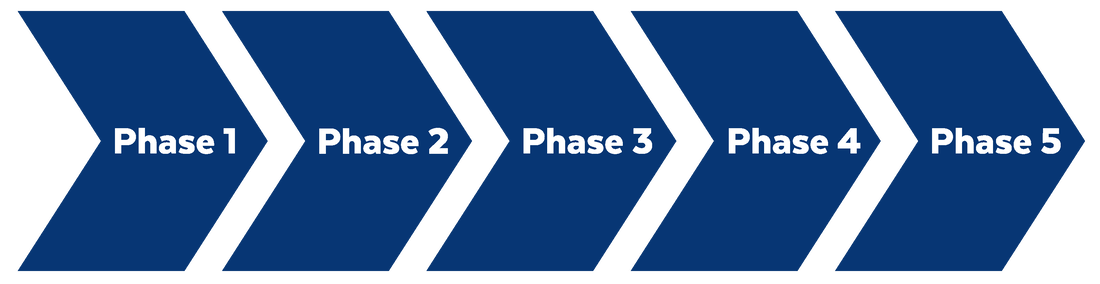
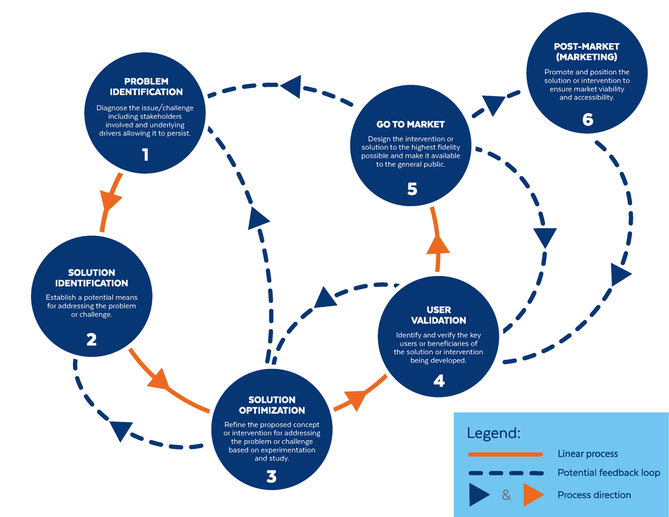
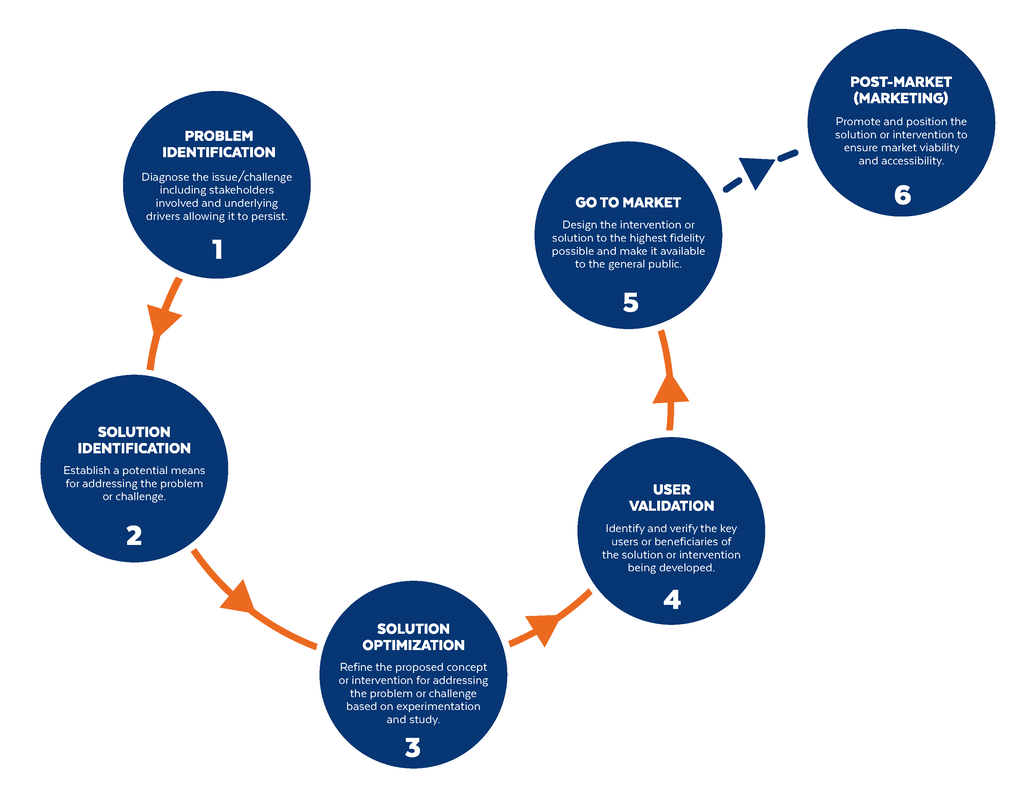
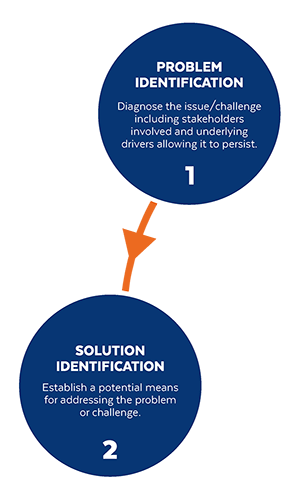
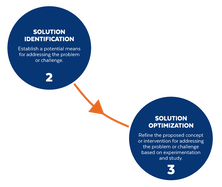
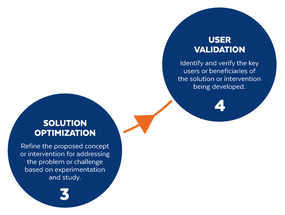
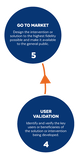
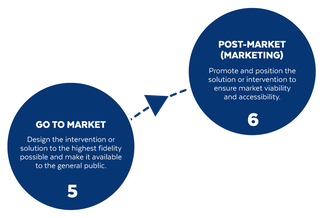
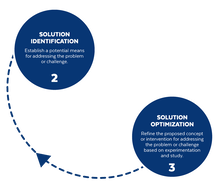
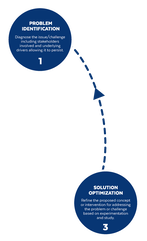
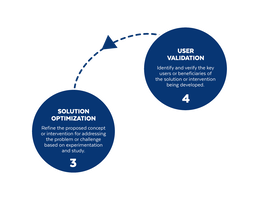
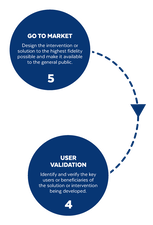
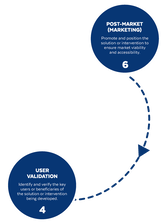
Based on the way that this information is conveyed, you might expect the process to visually represent something like this: But will the process of getting a new male contraceptive to market be such a clean, step-wise process? It’s a question that we wrestle with at Male Contraceptive Initiative. After all, the only male contraceptives on the market today are condoms and vasectomy; neither of which are “drugs”, so their regulatory journey to market was different than what many non-hormonal, reversible methods that we are supporting will likely face. With this in mind, we asked attendees to our inaugural ideation event to participate in a speculative design exercise seeking to help define and illustrate the anticipated journey to market for novel forms of male contraception. Given that participants represent a holistic sampling of subject matter experts from across the contraception research and development community, we feel that their collective knowledge and experience lends a certain level of gravitas to the exercise. That said, please keep in mind that this is speculative. The Swirling Vortex The immediate response from the group is that we need to consider the regulatory pathway as a “swirling vortex” full of feedback loops and interdependencies rather than a purely linear process. Granted, if, and this is a huge if, everything were to go perfectly as planned one could experience this journey in a linear fashion. However, it is highly unlikely that this can be achieved and should be viewed as an exception rather than the rule. The anticipated reality is that there will likely be multiple course-corrections along the way, utilizing an approach more akin to trial-and-error than one that is fully and clearly prescribed. Rather than visualizing this linearly, we synthesized the feedback from the group to create this illustration of the process: Viewed in toto, the vortex can be a bit intimidating as there are numerous connections and loops (necessary and/or potential) to contend with. The legend seeks to aid in unpacking the journey. The solid orange line seeks to highlight the step-by-step process that, in a perfect world, could represent the linear progression of a product from inception to market availability. The dotted blue line identifies the possible retrograde motions required should progress stall or fall short of expectations along the way. Steps Focusing solely on the individual pieces, the stepwise process emerges a bit more clearly: Each step is numbered, with the process beginning at #1 (Problem Identification) and continuing to the ideal “final” step #6 (Post-market/Marketing). (Step #6 is the last step when considering the process as a linear one. Ideally, this process is followed cyclically to ensure continued product viability and resonance.) The progression from step 5 to 6 is visually offset and indicated by a dotted line as it is part of the process beyond reaching market.
Transitions The transitions between each step represent distinct needs, opportunities, and challenges, that are important to keep in mind as you move through the process. Here we attempt to provide additional details to help elucidate these transitional realities.
Feedback Loops The real crux of the “Swirling Vortex” lies in the potential feedback loops necessary to ensure efficacy and value of drugs being developed. They represent the course corrections that a developer may need to make in order to effectively succeed at not only bringing a new drug to market, but ensuring that that drug maintains its impact throughout the course of its shelf life. Here we detail what each of these potential feedback loops entails.
Revisiting Target User(s)
Conclusion: Evolve or Die The Swirling Vortex is our attempt at articulating the potential process a male contraceptive solution may need to follow. As stated before, this is not prescriptive, nor is it a clean, linear journey with a clear beginning and end. Reaching the market is the ultimate goal, though it should not be indicative of work completing. The more a product can be re-tested, reevaluated, and refined the greater its long-term viability and resonance.
Comments are closed.
|
Categories
All
Archives
June 2024
|
|
|
Donate to Male Contraceptive InitiativeYour generous donation makes a difference!
|
© Male Contraceptive Initiative. All rights reserved.








 RSS Feed
RSS Feed
